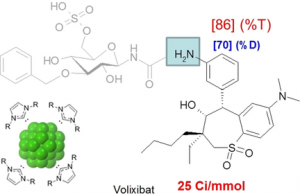
Valero et al. reported the preparation of N‐heterocyclic carbene‐stabilized iridium nanoparticles and their application in hydrogen isotope exchange reactions . These air‐stable and easy‐to‐handle iridium nanoparticles showed a unique catalytic activity, allowing selective and efficient hydrogen isotope incorporation on anilines using D2 or T2 as isotopic source. The usefulness of this transformation has been demonstrated by the deuterium and tritium labeling of diverse complex pharmaceuticals.
NHC‐Stabilized Iridium Nanoparticles as Catalysts in Hydrogen Isotope Exchange Reactions of Anilines
Mégane Valero, Dr. Donia Bouzouita, Dr. Alberto Palazzolo, Dr. Jens Atzrodt, Dr. Christophe Dugave, Dr. Simon Tricard, Dr. Sophie Feuillastre, Dr. Grégory Pieters, Dr. Bruno Chaudret, Dr. Volker Derdau
Angew. Chem. Int. Ed. 2020, 59, 3517 –3522. https://doi.org/10.1002/anie.201914369
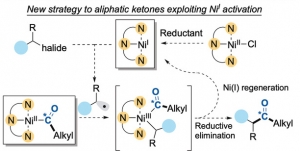
Skrydstrup et al. reported an extensive range of functionalized aliphatic ketones with good functional‐group tolerance prepared by a NiI‐promoted coupling of either primary or secondary alkyl iodides with NN2 pincer NiII‐acyl complexes. The latter were easily accessed from the corresponding NiII‐alkyl complexes with stoichiometric CO. This Ni‐mediated carbonylative coupling is adaptable to late‐stage carbon isotope labeling, as illustrated by the preparation of isotopically labelled pharmaceuticals. Preliminary investigations suggest the intermediacy of carbon‐centered radicals..
Direct Access to Isotopically Labeled Aliphatic Ketones Mediated by Nickel(I) Activation
Aske S. Donslund, Simon S. Pedersen, Cecilie Gaardbo, Dr. Karoline T. Neumann, Lee Kingston, Dr. Charles S. Elmore, Prof. Dr. Troels Skrydstrup
Angew. Chem. Int. Ed. 2020, https://doi.org/10.1002/anie.201914369
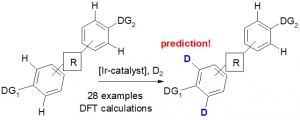
Derdau et al. reported an assessment of the C−H activation catalyst [(COD)Ir(IMes)(PPh3)]PF6 (COD=1,5‐cyclooctadiene, IMes=1,3‐bis(2,4,6‐trimethylphenyl)imidazol‐2‐ylidene) in the deuteration of phenyl rings containing different functional directing groups is divulged. Competition experiments have revealed a clear order of the directing groups in the hydrogen isotope exchange (HIE) with an iridium (I) catalyst. Through DFT calculations the iridium–substrate coordination complex has been identified to be the main trigger for reactivity and selectivity in the competition situation with two or more directing groups.
C−H Functionalization—Prediction of Selectivity in Iridium(I)‐Catalyzed Hydrogen Isotope Exchange Competition Reactions
Mégane Valero, Thomas Kruissink, Jennifer Blass, Remo Weck, Dr. Stefan Güssregen, Dr. Alleyn T. Plowright, Dr. Volker Derdau
Angew. Chem. Int. Ed. 2020, https://doi.org/10.1002/anie.201914369
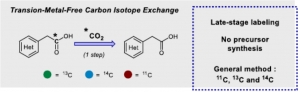
Audisio et al. reported Carbon Carbon Exchange in Phenylacetic acids without any Transition metal.
Transition-Metal-Free Carbon Isotope Exchange of Phenyl Acetic Acids
Gianluca Destro, Kaisa Horkka, Olivier Loreau, David-Alexandre Buisson, Lee Kingston, Antonio Del Vecchio, Magnus Schou, Charles Elmore, Frédéric Taran, Thibault Cantat, and Davide Audisio
Angew. Chem. Int. Ed. 2020, https://doi.org/10.1002/anie.202002341